Malek Alnajar
College of Nursing, The University of Utah
NURS 7106- Context for Advancing Science
Dr. Deanna Kepka
April 25, 2023
Fecal microbiome transplant (FMT) is a therapeutic strategy that relies on
transferring fecal microbiota extracted from the feces of one healthy individual donor to a diseased recipient. Transplantation is done by endoscopy, enema, or oral administration. The new bacterial populations regain the depleted flora's assumed protective and regulatory functions. In the past, FMT was commonly used to treat clostridium difficile (C. difficile) infections, commonly affecting individuals on long-term antibiotic therapies that may drain the normal flora of the intestines. Recently, emerging evidence is proving the effectiveness of FMT in improving neurologic symptoms and thus slowing the disease progression.
Microbiota in the Human Body
The human microbiome, often called the virtual organ, is the collection of bacteria
that colonizes the human body. This microbiota is believed to be introduced to the body for
the first time during the early stages of life, either before or during delivery through contact with maternal microbial colonization, and then proliferates and diverse to resemble that found in an adult body within the first 2.5 years of life. The microbiota has a vital role in the body's developmental, nutritional, and immunological functions, thus highly affecting health and disease status.
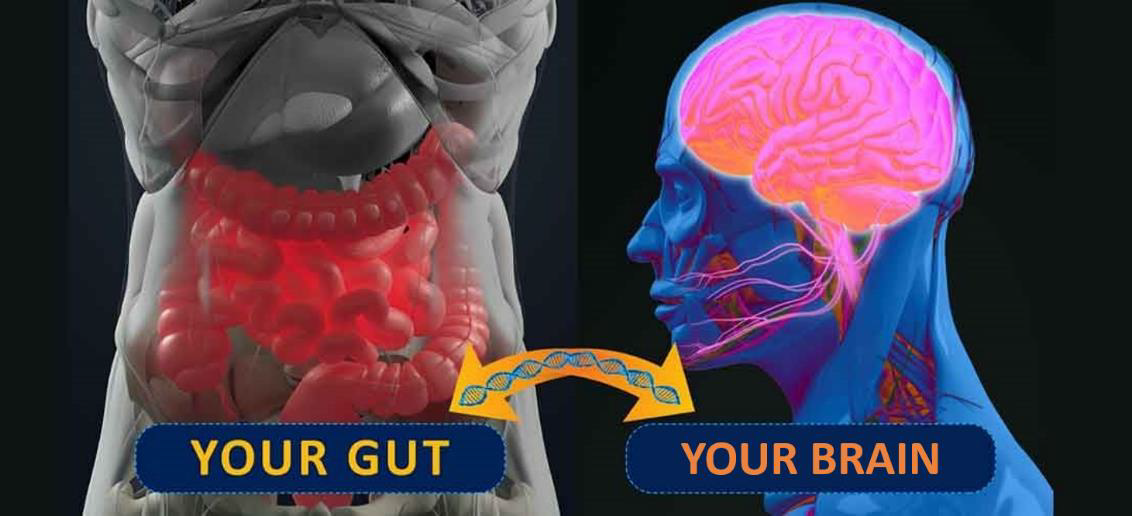
The gastrointestinal tract (GIT) harbors the most microbiota in the human body. The gut microbiota has a significant role in developing different body systems, including the central nervous system (CNS) and the autonomic nervous system (ANS), where its genetic material aids in regulating brain development and function. This works through the gut-brain
axis (GBA). GBA is a two-way signalling system between the gut and the brain that uses
endocrine, neural, and immune regulators for communication.
Microbiota varies from one individual to another depending on many factors, such as diet, age, genetics, metabolism, geography, antibiotic treatment, illness, stress, and even mode of delivery. These factors interact with the gut altering its function in regulating body mechanisms, including brain function. Changes in microbiota status directly change different neurologic symptoms such as anxiety, stress, and other behavioral and mood disorders.
Microbiota and Neurologic Symptoms
Different literature proved the relationship between the gut microbiome function and neurologic symptoms of multiple neurodegenerative disorders such as irritable bowel syndrome (IBS), Multiple sclerosis (MS), Alzheimer’s disease (AD), and Parkinson's disease (PD). Neurodegenerative disorders are varied and directly affect the daily functioning of affected patients. These disorders underly a varied spectrum of symptoms, such as abdominal pain, irregular defecation patterns, abdominal distension and discomfort, stiffness, bradykinesia, and tremors. Since these are noncurative disorders, symptom management is a
priority. Different modalities have been used to control these symptoms for years; pharmacologic (e.g., probiotics, antibiotics, dopamine replacement, etc.) and non- pharmacologic (e.g., exercise therapy, dietary modifications, etc.) interventions have proved their effectiveness in providing symptom control. However, using these modalities is associated with potential risks and leads to undesired side effects, as well as the social stigma associated with psychiatric medication use. In light of these circumstances, the need to find new management is urgent to improve the health outcomes of these patients. FMT significantly enhances the daily functioning of affected individuals as it improves the function of the GBA and decreases restrictive uncomfortable symptoms in the short term.
Effectiveness of FMT in Managing Neurologic Symptoms:
FMT has proved to have better curative outcomes in treating gut infections than antibiotics for years now without any reported adverse effects. Recently, a case report of a MS patient reported over ten years of stability in neurologic symptoms after a FMT that was used for constipation. Others suggested that FMT has stopped the disease progression and led to some impairment in the neurons that were not seen with previous interventions. For patients with IBS, previous therapies did not offer satisfactory outcomes in the long run, while FMT showed positive outcomes for more than 25 weeks. FMT has also demonstrated a vast alteration in the microbiome for patients with PD than that seen with antibiotics or probiotics. Such progress was a lack in previous treatments and did not last as long as the results of FMT did (almost six months). On the other hand, some clinical studies reported a relapse in these neurologic symptoms in the long term (more than 12 weeks of transplant) among patients with IBS.
Challenges with FMT are related to the reported adverse events associated with the treatment. Short-term events such as bloating, abdominal pain, fluctuation in bowel habits,
fever, nausea, and belching are considered acceptable compared to the benefits gained with the treatment. Long-term adverse events reflected increased disease vulnerability that is triggered by the donor microbiome, reports of obesity and immune-mediated disorders like thrombocytopenia, rheumatoid arthritis, and inflammatory bowel syndrome were recorded. The risk of transmitting infection and developing a new infectious disease within one to six months of the transplant was also highlighted. Aspiration pneumonia was mentioned as an adverse event associated with the route of treatment delivery. The risk is established with the upper gastrointestinal route through nasoduodenal or nasojejunal routes.
To overcome these challenges, cautious donor selection and microbiome screening are explicitly required for multi-drug resistant organisms. Much theoretical research supports
using FMT to manage neurologic symptoms, but clinical trials among human subjects are still limited. More research in this area is needed to better understand its effectiveness, safety, and mechanism of action.
Future of FMT:
Based on the massive effect of microbiota on human health status, it is consistently investigated for future therapeutic directions to manage a wide variety of conditions like, cancer, metabolic syndrome, liver and pancreatic disease, and autism spectrum disorders, among many others. FMT currently focuses on clinical research and is expected to show promising results and applications.
Here is a recommended video to watch: https://www.youtube.com/watch?v=C9bYKd_Ffgc
References
American Psychiatric Association. (2013). Depressive disorders and anxiety disorders. In: Diagnostic and statistical manual of mental disorders. 5th ed. Philadelphia: American Psychiatric Association. p. 93–128.
Chinna Meyyappan, A., Forth, E., Wallace, C. J., & Milev, R. (2020). Effect of fecal microbiota transplant on symptoms of psychiatric disorders: a systematic review. BMC psychiatry, 20(1), 1-19.
Cryan, J. F., & Dinan, T. G. (2012). Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nature reviews neuroscience, 13(10), 701-712.
Cussotto, S., Clarke, G., Dinan, T. G., & Cryan, J. F. (2019). Psychotropics and the microbiome: a chamber of secrets…. Psychopharmacology, 236, 1411-1432.
Dash, S., Clarke, G., Berk, M., & Jacka, F. N. (2015). The gut microbiome and diet in psychiatry: focus on depression. Current opinion in psychiatry, 28(1), 1-6.
Foster, J. A., & Neufeld, K. A. M. (2013). Gut–brain axis: how the microbiome influences anxiety and depression. Trends in neurosciences, 36(5), 305-312.
Huang, H., Xu, H., Luo, Q., He, J., Li, M., Chen, H., ... & Zhou, Y. (2019). Fecal microbiota transplantation to treat Parkinson's disease with constipation: a case
report. Medicine, 98(26).
Makkawi, S., Camara-Lemarroy, C., & Metz, L. (2018). Fecal microbiota transplantation associated with 10 years of stability in a patient with SPMS. Neurology- Neuroimmunology Neuroinflammation, 5(4).
Matheson, J. A. T., & Holsinger, R. D. (2023). The role of fecal microbiota transplantation in the treatment of neurodegenerative diseases: A review. International Journal of Molecular Sciences, 24(2), 1001..
Mazzawi, T., Lied, G. A., Sangnes, D. A., El-Salhy, M., Hov, J. R., Gilja, O. H., ... & Hausken, T. (2018). The kinetics of gut microbial community composition in patients with irritable bowel syndrome following fecal microbiota transplantation. PloS
one, 13(11), e0194904.
Park, S. Y., & Seo, G. S. (2021). Fecal microbiota transplantation: is it safe?. Clinical Endoscopy, 54(2), 157-160.
Tkach, S., Dorofeyev, A., Kuzenko, I., Boyko, N., Falalyeyeva, T., Boccuto, L., ... & Abenavoli, L. (2022). Current status and future therapeutic options for fecal microbiota transplantation. Medicina, 58(1), 84.


No comments:
Post a Comment